
What are the major sources of microbial contamination in a cleanroom?
Share
February 7th, 2023. Hannu Karhu, CEO & Cleanroom specialist, Abonano. Tuukka Autio, Product management, LED Tailor.
This article gives you a concise overview of how microbial contamination can enter your cleanroom and what you can do about it.
Article contents:
- Why and how contamination is controlled in a cleanroom
- What are the major sources of contamination in a cleanroom
a) People flow
b) Material flow
c) Air flow - Microbe flora typically found in cleanrooms
- An automatic way to prevent and control contamination in a cleanroom
1. Why and how contamination is controlled in a cleanroom
Users of cleanrooms with ISO or GMP classification, for example in the manufacture of pharmaceutical products or medical devices, must consider the potential sources and risks of microbial contamination in their production processes.
Since contaminated drugs or medical devices can potentially cause life-threatening infections or other complications, the industry is highly regulated and manufacturers must comply with strict standards and continuously measure and control their facilities’ contamination levels.
The strategies for preventing and controlling microbial contamination must take into account the whole manufacturing process, from the sourcing and handling of raw material supplies to preparation and production, and further to packaging and storage of ready products.
The tools and practices in contamination prevention and control have traditionally focused on ventilation, personal protective cleanroom apparel, and thorough cleaning and chemical disinfection of surfaces.
However, regardless of even the best traditional counter measures and strategies, microbial contamination is sometimes still found in cleanrooms and causes a lot of extra work and high costs to the industry. New automatic methods to prevent contamination and reduce the associated costs are therefore highly desired.
2. What are the major sources of contamination in a cleanroom?
Practical experience and academic research on cleanrooms suggest that we can divide the most common sources of microbial contamination in a cleanroom into three main categories:
a) People flow
b) Material flow – solid materials and water
c) Air flow and ventilation
Dr. Tim Sandle (2011, 2022) has studied and summarized the division of different sources of contamination in several publications and presentations (see pie chart below). The actual percentage will vary across different facilities and types of manufacture, but this can be used as a general guidance.

Chart 1: Most common sources of microbial contamination in cleanrooms (Tim Sandle, 2022)
a) People flow (~70%): According to several studies and industry surveys, the majority of microbes found in cleanrooms are human-associated and can be traced back to the gowning areas. As a consequence of people working and moving in the cleanroom, microbes naturally living on the surface of people’s skin and microbes that people exhale can enter the cleanroom.
Preventing particles and microbes is a constant challenge associated with personnel entering the cleanroom. How the gowning rooms (airlocks) are organized and how cleanroom clothes are put on in the gowning rooms have an impact on particle and microbe emissions from personnel in the cleanroom.
In general, limiting the number of people entering the cleanroom and minimizing the time spent there can be one of the most impactful measures in preventing microbial contamination related to people flow.
b) Material flow, including water (~5-15%): Raw materials and other supplies, or their packaging materials, coming in to the cleanroom facility can be a major source of contamination.
According to studies, microbial contamination related to materials can frequently be traced to bags, boxes, especially carton boxes; pallets, trolley wheels, shoes and shoe covers. Dr. Sandle also lists door kick panels as a frequent source of contamination.
The outer layers of packaging are routinely discarded step by step when materials are moved from the storage to cleanroom preparation and production areas. The unboxing can agitate particles and microbes and make them airborne, spreading them around the room and its surfaces. The personnel’s shoes and the wheels of trolleys can also pick up and carry microbes and microbial spores from the dirtier area to the cleaner area.
If microbes can get into the cleanroom with material flows, there is a risk that contaminated products can get out of the facility too. The transport of items in and out of the clean areas can account for roughly 5% of microbial contamination, but naturally this percentage can vary based on the nature of the production and how well the transport processes can be controlled.
- In addition to solid materials, water is also often used in cleanroom processes. Water tanks and pipes can over time become sources of contamination, and production areas where water is used may have moisture or spills that can promote microbial growth. The forming of biofilm is often associated with containers or areas where water has been stored or handled. According to studies, water can account for up to 10% of microbial contamination.
c) Air flow and ventilation: Controlling the purity of the air in a cleanroom is a critical factor, because airborne particles and microbes land on materials, surfaces, equipment and products.
Using HEPA or ULPA filters, and in many cases pressure differences between cleanroom and surrounding area, the air in the cleanroom is kept as free of particles and microbes as deemed necessary. The level of air purity requirement (ISO/cGMP class) is based on risk analysis and regulation.
If the cleanroom filter/HVAC system is not functioning fully as intended because of HEPA/ULPA filter failures and/or unintended airflow from airlocks to production area, a larger number of particles and microbes may enter the production area. In one example, a production facility had mold growth in their HEPA filters.
Roughly 10% of contamination can be attributed to air flows. In case of filter or airlock malfunction the rise in contamination can be substantial.
3. Microbe flora found in cleanrooms based on our experiences
Based on our experience and our customer surveys, the following microbes are among the most commonly seen examples of bioburden in GMP cleanrooms. The list is not exhaustive, and many other microbes can be found depending on the nature of manufacturing.
- "Bacterial spores on product packaging"
- "Bacillus spores"
- "Bacillus", "Bacillus subtilis", "Bacillus cereus"
- "Staphylococcus" (different species of)
- "Escherichia coli"
- "Aspergillus brasiliensis"
- "Pseudomonas aeruginosa"
- "Micrococcus luteus"
In food processing plants and their clean production areas the microbe problems our customers have reported have been with the following microbes:
- "Listeria"
- "Escherichia coli"
- "Salmonella"
- "Mold + spores"
4. An automatic way to prevent and control contamination in a cleanroom
With such many potential pathways for microbes to enter a cleanroom, the importance of frequent and thorough cleaning and disinfection of surfaces is evident.
Spectral Blue is a new touch-free method for automatic disinfection of air and surfaces in cleanrooms. It uses visible blue light to kill microbes. Because it’s UV-free and chemical-free, it’s completely safe for people and materials and can be used in all areas.
Our recommended response to microbial contamination risks associated with people flows is to minimize the time spent in the cleanroom and to deploy automatic blue light disinfection in gowning areas and personnel airlocks. Blue light can provide continuous surface disinfection and prevent microbial growth between cleaning rounds.
To reduce risks associated with material flows, we recommend treating materials and equipment with blue light disinfection prior to entering the cleanroom production area. Material airlocks, material storage areas and equipment storage areas are key locations for installing a blue light system.
The production and packaging areas are treated with automatic blue light disinfection continuously or daily, whenever possible.
Despite traditional approaches and extensive regulation to prevent microbial contamination in cleanrooms, sometimes hygiene failures still occur and can lead to production halts, documentation work, corrective actions, product recalls and other costs of non-compliance.
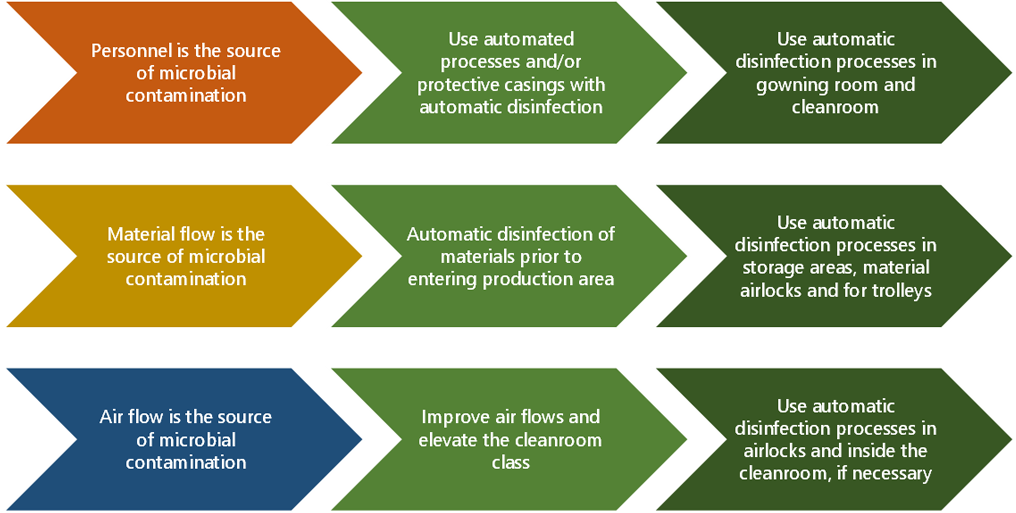
Chart 2: Contamination prevention and control strategies in a cleanroom
Automatic disinfection measures like Spectral Blue complement manual decontamination practices and effectively and in repeatable manner reduce the risk of hygiene failures and associated high costs. If the use of chemical disinfectants can be reduced at the same time, the sustainability of the whole value chain and healthiness of the working environment is improved – which is an important goal for both management and personnel.
- Interested in how the Spectral Blue technology works? Please see our science section for a video and articles. Also be sure to see our section about solutions for cleanrooms. And if you’d like to test it in your facilities, contact us and we’ll help you to get started in no time.
References:
Sandle, Tim. A Review of Cleanroom Microflora: Types, Trends, and Patterns. PDA J Pharm Sci and Tech 2011, 65 392-403.
Sandle, Tim. Contamination Control Strategy and Rapid Microbiological Methods. Online presentation 2022 (link to video).
Related articles in our news section:
- "Substantial and persistent reduction of microbial burden in a personnel cut-off room within a pharmaceutical facility following the implementation of automated and continuous Spectral Blue™ disinfection"
- NextPharma: "Using Spectral Blue disinfection in cleanroom boundary areas reduced contamination in production areas by 90%"'
- Phillips-Medisize (Molex Group): "80% reduction in manual disinfection thanks to blue light"
Also watch our cleanroom webinar (Autumn 2024): Video recording is available here.
